Microcirculation – the flow of blood through the smallest vessels – is responsible for distributing oxygen and nutrients to tissues and organs throughout the body. Mapping this flow at the whole-organ scale could enhance our understanding of the circulatory system and improve diagnosis of vascular disorders. With this aim, researchers at the Institute Physics for Medicine Paris (Inserm, ESPCI-PSL, CNRS) have combined 3D ultrasound localization microscopy (ULM) with a multi-lens array method to image blood flow dynamics in entire organs with micrometric resolution, reporting their findings in Nature Communications.
“Beyond understanding how an organ functions across different spatial scales, imaging the vasculature of an entire organ reveals the spatial relationships between macro- and micro-vascular networks, providing a comprehensive assessment of its structural and functional organization,” explains senior author Clement Papadacci.
The 3D ULM technique works by localizing intravenously injected microbubbles. Offering a spatial resolution roughly ten times finer than conventional ultrasound, 3D ULM can map and quantify micro-scale vascular structures. But while the method has proved valuable for mapping whole organs in small animals, visualizing entire organs in large animals or humans is hindered by the limitations of existing technology.
To enable wide field-of-view coverage while maintaining high-resolution imaging, the team – led by PhD student Nabil Haidour under Papadacci’s supervision – developed a multi-lens array probe. The probe comprises an array of 252 large (4.5 mm²) ultrasound transducer elements. The use of large elements increases the probe’s sensitive area to a total footprint of 104 x 82 mm, while maintaining a relatively low element count.
Each transducer element is equipped with an individual acoustic diverging lens. “Large elements alone are too directive to create an image, as they cannot generate sufficient overlap or interference between beams,” Papadacci explains. “The acoustic lenses reduce this directivity, allowing the elements to focus and coherently combine signals in reception, thus enabling volumetric image formation.”
Whole-organ imaging
After validating their method via numerical simulations and phantom experiments, the team used a multi-lens array probe driven by a clinical ultrasound system to perform 3D dynamic ULM of an entire explanted porcine heart – considered an ideal cardiac model as its vascular anatomies and dimensions are comparable to those of humans.
The heart was perfused with microbubble solution, enabling the probe to visualize the whole coronary microcirculation network over a large volume of 120 x 100 x 82 mm, with a spatial resolution of around 125 µm. The technique enabled visualization of both large vessels and the finest microcirculation in real time. The team also used a skeletonization algorithm to measure vessel radii at each voxel, which ranged from approximately 75 to 600 µm.
As well as structural imaging, the probe can also assess flow dynamics across all vascular scales, with a high temporal resolution of 312 frames/s. By tracking the microbubbles, the researchers estimated absolute flow velocities ranging from 10 mm/s in small vessels to over 300 mm/s in the largest. They could also differentiate arteries and veins based on the flow direction in the coronary network.
In vivo demonstrations
Next, the researchers used the multi-lens array probe to image the entire kidney and liver of an anaesthetized pig at the Veterinary school of Maison Alfort, with the probe positioned in front of the kidney or liver, respectively, and held using an articulated arm. They employed electrocardiography to synchronize the ultrasound acquisitions with periods of minimal respiratory motion and injected microbubble solution intravenously into the animal’s ear.
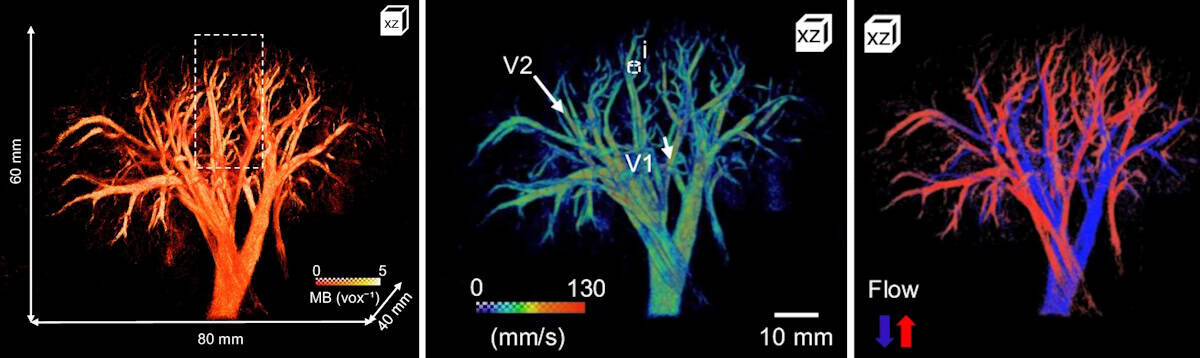 <><><><><><>
<><><><><><>The probe mapped the vascular network of the kidney over a 60 x 80 x 40 mm volume with a spatial resolution of 147 µm. The maximum 3D absolute flow velocity was approximately 280 mm/s in the large vessels and the vessel radii ranged from 70 to 400 µm. The team also used directional flow measurements to identify the arterial and venous flow systems.
Liver imaging is more challenging due to respiratory, cardiac and stomach motions. Nevertheless, 3D dynamic ULM enabled high-depth visualization of a large volume of liver vasculature (65 x 100 x 82 mm) with a spatial resolution of 200 µm. Here, the researchers used dynamic velocity measurement to identify the liver’s three blood networks (arterial, venous and portal veins).
“The combination of whole-organ volumetric imaging with high-resolution vascular quantification effectively addresses key limitations of existing modalities, such as ultrasound Doppler imaging, CT angiography and 4D flow MRI,” they write.
Clinical applications of 3D dynamic ULM still need to be demonstrated, but Papadacci suggests that the technique has strong potential for evaluating kidney transplants, coronary microcirculation disorders, stroke, aneurysms and neoangiogenesis in cancer. “It could also become a powerful tool for monitoring treatment response and vascular remodelling over time,” he adds.
Papadacci and colleagues anticipate that translation to human applications will be possible in the near future and plan to begin a clinical trial early in 2026.
The post Ultrasound probe maps real-time blood flow across entire organs appeared first on Physics World.

