Neurodegenerative diseases affect millions of people worldwide, but treatment of such conditions is limited by the blood–brain barrier (BBB), which blocks the passage of drugs to the brain. In the quest for more effective therapeutic options, a multidisciplinary research team has developed a novel machine learning-based technique to predict the behaviour of nanoparticles as drug delivery systems.
The work focuses on nanoparticles that can cross the BBB and provide a promising platform for enhancing drug transport into the brain. But designing specific nanoparticles to target specific brain regions is a complex and time-consuming task; there’s a need for improved design frameworks to identify potential candidates with desirable bioactivity profiles. For this, the team – comprising researchers from the University of the Basque Country (UPV/EHU) in Spain and Tulane University in the USA, led by the multicentre CHEMIF.PTML Lab – turned to machine learning.
Machine learning uses molecular and clinical data to detect trends that may lead to novel drug delivery strategies with improved efficiency and reduced side effects. In contrast to slow and costly trial-and-error or physical modelling approaches, machine learning could provide efficient initial screening of large combinations of nanoparticle compositions. Traditional machine learning, however, can be hindered by the lack of suitable data sets.
To address this limitation, the CHEMIF.PTML Lab team developed the IFE.PTML method – an approach that integrates information fusion, Python-based encoding and perturbation theory with machine learning algorithms, describing the model in Machine Learning: Science and Technology.
“The main advantage of our IFE.PTML method lies in its ability to handle heterogeneous nanoparticle data,” corresponding author Humberto González-Díaz explains. “Standard machine learning approaches often struggle with disperse and multi-source datasets from nanoparticle experiments. Our approach integrates information fusion to combine diverse data types – such as physicochemical properties, bioassays and so on – and applies perturbation theory to model these uncertainties as probabilistic perturbations around baseline conditions. This results in more robust, generalizable predictions of nanoparticle behaviour.”
To build the predictive models, the researchers created a database containing physicochemical and bioactivity parameters for 45 different nanoparticle systems across 41 different cell lines. They used these data to train IFE.PTML models with three machine learning algorithms – random forest, extreme gradient boosting and decision tree – to predict the drug delivery behaviour of various nanomaterials. The random forest-based model showed the best overall performance, with accuracies of 95.1% and 89.7% on training and testing data sets, respectively.
Experimental demonstration
To illustrate the real-world applicability of the random forest-based IFE.PTML model, the researchers synthetized two novel magnetite nanoparticle systems (the 31 nm-diameter Fe3O4_A and the 26 nm-diameter Fe3O4_B). Magnetite-based nanoparticles are biocompatible, can be easily functionalized and have a high surface area-to-volume ratio, making them efficient drug carriers. To make them water soluble, the nanoparticles were coated with either PMAO (poly(maleic anhydride-alt-1-octadecene)) or PMAO plus PEI (poly(ethyleneimine).
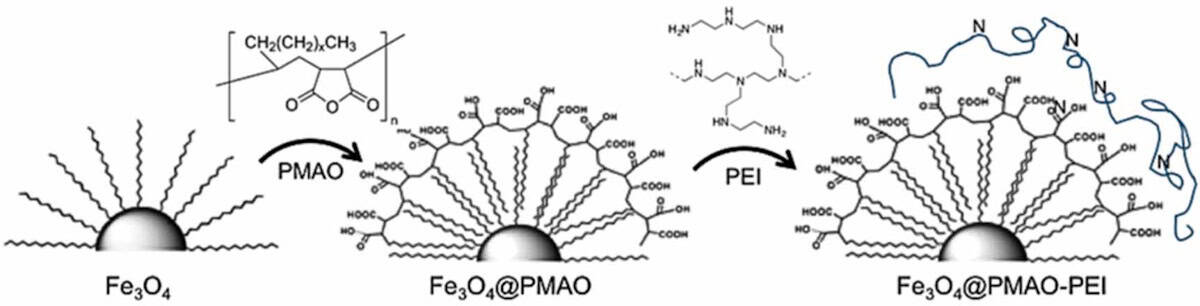 <><><><><><><><>
<><><><><><><><>The team characterized the structural, morphological and magnetic properties of the four nanoparticle systems and then used the optimized model to predict their likelihood of favourable bioactivity for drug delivery in various human brain cell lines, including models of neurodegenerative disease, brain tumour models and a cell line modelling the BBB.
As inputs for their model, the researchers used a reference function based on the bioactivity parameters for each system, plus perturbation theory operators for various nanoparticle parameters. The IFE.PTML model calculated key bioactivity parameters, focusing on indicators of toxicity, efficacy and safety. These included the 50% cytotoxic, inhibitory, lethal and toxic concentrations (at which 50% of the biological effect is observed) and the zeta potential, which affects the nanoparticles’ capacity to cross the BBB. For each parameter, the model output a binary result: “0” for undesired and “1” for desired bioactivities.
The model identified PMAO-coated nanoparticles as the most promising candidates for BBB and neuronal applications, due to their potentially favourable stability and biocompatibility. Nanoparticles with PMAO-PEI coatings, on the other hand, could prove optimal for targeting brain tumour cells.
The researchers point out that, where comparisons were possible, the trends predicted by the RF-IFE.PTML model agreed with the experimental findings, as well as with previous studies reported in the literature. As such, they conclude that their model is efficient and robust and offers valuable predictions on nanoparticle–coating combinations designed to act on specific targets.
“The present study focused on the nanoparticles as potential drug carriers. Therefore, we are currently implementing a combined machine learning and deep learning methodology with potential drug candidates for neurodegenerative diseases,” González-Díaz tells Physics World.
- CHEMIF.PTML Lab is a multicentre laboratory of the Biofisika Institute of UPV/EHU and the Spanish National Research Council, supported by Ikerbasque, the Basque Foundation for Science.
The post Machine learning optimizes nanoparticle design for drug delivery to the brain appeared first on Physics World.

