
In what has been a long period of relative low activity, FDA’s OPDP has taken the opportunity to remind us that low enforcement does not mean no enforcement when it comes to promotional speech by pharmaceutical companies.
This past June FDA posted an Untitled Letter, the first regulatory action letter in a year, reported on here regarding a website communication. This month FDA posted another letter, this one a Warning Letter involving a Sales Aid. This second letter of 2023 makes a total of six letters for the combined years of 2022-2023, two of which were Warning Letters and four of which were Untitled.
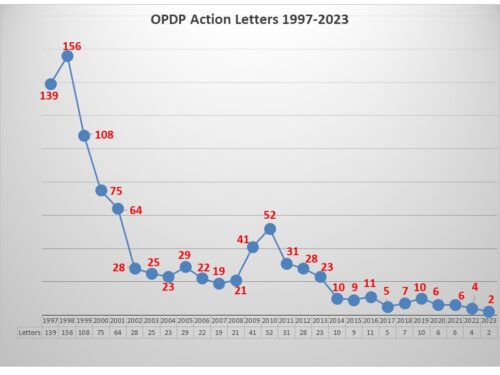
With so little activity, trends are often difficult to discern, but one does appear to emerge.
In examining four of the last six regulatory action letters issued by OPDP, one discerns that there is possibly an emergent sensitivity the agency has in looking at communications that make claims about a product, even when citing published papers as evidence.
The last four letters from OPDP:
- August 4, 2023 – Warning Letter – Sales AidJune 7, 2023 – Untitled Letter – Website June 2, 2022 – Untitled Letter – Doctor LetterMarch 31, 2022 – Untitled Letter – Web page
Each of these letters have a common thread – in each of the last four letters issued by OPDP the agency was taking issue with the support that existed for efficacy claims being made in the communication about the medicine. In each situation, OPDP closely analyzed the studies for their applicability to the claim being made, assessing whether or not they were adequate to the task from the perspective of the agency.
With four in a row, that would seem to signal that promotional communications that deal with efficacy claims may be speech that is of special interest to the agency right now, and that enforcement actions are sending that signal. Future letters will indicate if this persists as a point of interest.
By the way, for those who want to follow or examine the letters, FDA now separates the reporting of Untitled Letters from Warning Letters. To view the former, you can go to this page and see the Untitled Letters issued each year. If you want to see Warning Letters, you must go the Warning Letters page and perform a search specific to the issuing office – Office of Prescription Drug Promotion.
Photo by Goh Rhy Yan at Unsplash

