A combination of static proton arcs and shoot-through proton beams could increase plan conformity and homogeneity and reduce delivery times in upright proton therapy, according to new research from RaySearch Laboratories in Sweden.
Proton arc therapy (PAT) is an emerging rotational delivery technique with potential to improve plan quality – reducing dose to organs-at-risk while maintaining target dose. The first clinical PAT treatments employed static arcs, in which multiple energy layers are delivered from many (typically 10 to 30) discrete angles. Importantly, static arc PAT can be delivered on conventional proton therapy machines. It also offers simpler beam arrangements than intensity-modulated proton therapy (IMPT).
“In IMPT of head-and-neck cancers, the beam directions are normally set up in a complicated pattern in different planes, with range shifters needed to treat the shallow part of the tumour,” explains Erik Engwall, chief physicist at RaySearch Laboratories. “In PAT, the many beam directions are arranged in the same plane and no range shifters are typically needed. With all beams in the same plane, it is easier to move to upright treatments.”
Upright proton therapy involves rotating the patient (in an upright position) in front of a static horizontal treatment beam. The approach could reduce costs by using compact proton delivery systems. This compactness, however, places energy selection close to the patient, increasing scattering in the proton beam. To combat this, the team propose adding a layer of shoot-through protons to each direction of the proton arc.
The idea is that while most protons are delivered with Bragg peaks placed in the target, the sharp penumbra of the high-energy protons shooting through the target will combat beam broadening. The rotational delivery in the proton arc spreads the exit dose from these shoot-through beams over many angles, minimizing dose to surrounding tissues. And as the beamline is fixed, shoot-through protons exit in the same direction (behind the patient) for all angles, simplifying shielding to a single beam dump opposite the fixed beam.
Simulation studies
To test this approach, Engwall and colleagues simulated treatment plans for a virtual phantom containing three targets and an organ-at-risk, reporting their findings in Medical Physics. They used a development version of RayStation v2025 with a beam model of the Mevion s250-FIT system (which combines a compact cyclotron, an upright positioner and an in-room CT scanner).
For each target, the team created static arc plans with (Arc+ST) and without shoot-through beams and with/without collimation, as well as 3-beam IMPT plans with and without shoot-through beams (all with collimation). Arc plans used 20 uniformly spaced beam directions, and the shoot-through plans included an additional layer of the highest system energy (230 MeV) for each direction.
For all targets, Arc+ST plans showed superior conformity, homogeneity and target robustness to arc plans without shoot-through protons. Adding collimation slightly improved the arc plans without shoot-through protons but had little impact on Arc+ST plans.
The IMPT plans achieved similar homogeneity and robustness to the best arc plans, but with far lower conformity due to the shoot-through protons delivering a concentrated exit dose behind the target (while static arcs distribute this dose over many directions). Adding shoot-through protons improved IMPT plan quality, but to a lesser degree than for PAT plans.
Clinical case
The researchers repeated their analysis for a clinical head-and-neck cancer case, comparing static arcs with 5-beam IMPT. Again, Arc+ST plans performed better than any others for almost all metrics. “The Arc+ST plans have the best quality due to the sharpening of the penumbra of the shoot-through part, even better than when using a collimator,” says Engwall.
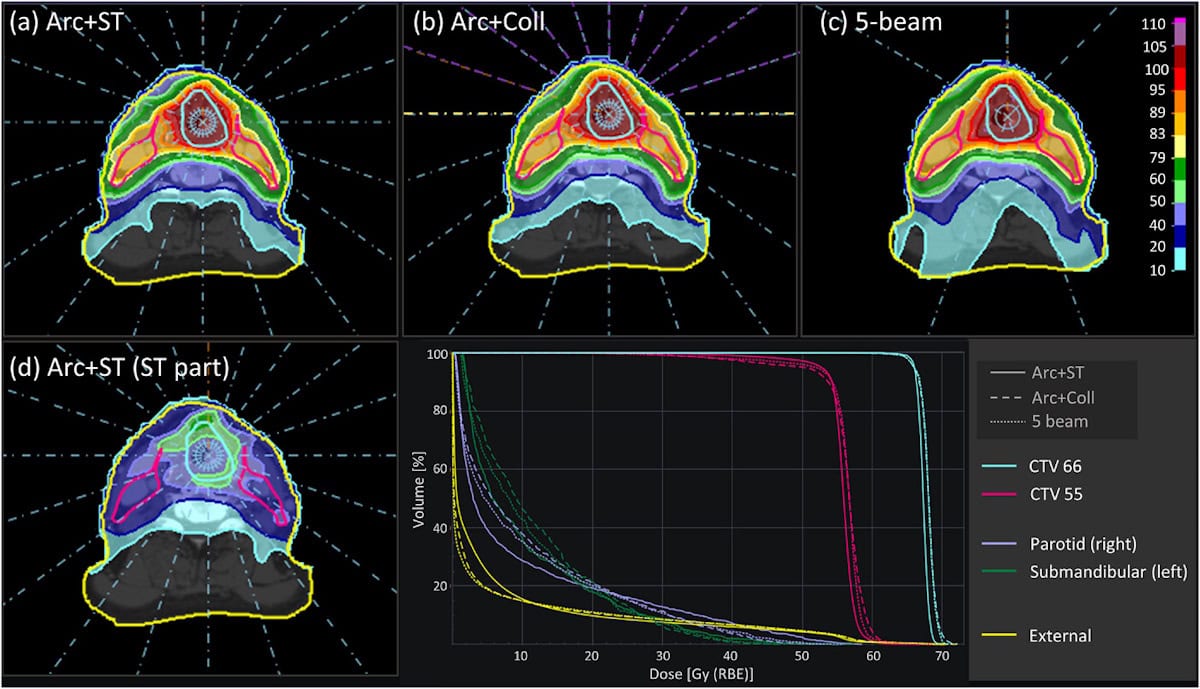 <><>–<><>
<><>–<><>Notably, the findings suggest that collimation is not needed when combining arcs with shoot-through beams, enabling rapid treatments. With fast energy switching and the patient rotation at 1 rpm, Arc+ST achieved an estimated delivery time of less than 5.4 min – faster than all other plans for this case, including 5-beam IMPT.
“Treatment time is reduced when the leaves of the dynamic collimator do not need to move,” Engwall explains. “There is also no risk of mechanical failures of the collimator and the secondary neutron production will be lower when there are fewer objects in the beamline.”
Another benefit of upright delivery is that the shoot-through protons can be used for range verification during treatments, using a detector integrated into the beam dump behind the patient. The team investigated this concept with three simulated error scenarios: 5% systematic shift in stopping power ratio; 5 mm setup shift; and 2 cm shoulder movement. The technique successfully detected all errors.
As the range detector is permanently installed in the treatment room and the shoot-through protons are part of the treatment plan, this method does not add time to the patient setup and can be used in every treatment fraction to detect both intra- and inter-fraction uncertainties.
Although this is a proof-of-concept study, the researchers conclude that it highlights the combined advantages of the new treatment technique, which could “leverage the potential of compact upright proton treatments and make proton treatments more affordable and accessible to a larger patient group”.
Engwall tells Physics World that the team is now collaborating with several clinical research partners to investigate the technique’s potential across larger patient data sets, for other treatment sites and multiple treatment machines.
The post Optimizing upright proton therapy: hybrid delivery provides faster, sharper treatments appeared first on Physics World.

