Researchers in Germany have demonstrated the first cancer treatment using a radioactive carbon ion beam (11C), on a mouse with a bone tumour close to the spine. Performing particle therapy with radioactive ion beams enables simultaneous treatment and visualization of the beam within the body.
Particle therapy using beams of protons or heavy ions is a highly effective cancer treatment, with the favourable depth–dose deposition – the Bragg peak – providing extremely conformal tumour targeting. This conformality, however, makes particle therapy particularly sensitive to range uncertainties, which can impact the Bragg peak position.
One way to reduce such uncertainties is to use positron emission tomography (PET) to map the isotopes generated as the treatment beam interacts with tissues in the patient. For therapy with carbon (12C) ions, currently performed at 17 centres worldwide, this involves detecting the beta decay of 10C and 11C projectile fragments. Unfortunately, such fragments generate a small PET signal, while their lower mass shifts the measured activity peak away from the Bragg peak.
The researchers – working within the ERC-funded BARB (Biomedical Applications of Radioactive ion Beams) project – propose that treatment with positron-emitting ions such as 11C could overcome these obstacles. Radioactive ion beams have the same biological effectiveness as their corresponding stable ion beams, but generate an order of magnitude larger PET signal. They also reduce the shift between the activity and dose peaks, enabling precise localization of the ion beam in vivo.
“Range uncertainty remains the main problem of particle therapy, as we do not know exactly where the Bragg peak is,” explains Marco Durante, head of biophysics at the GSI Helmholtz Centre for Heavy Ion Research and principal investigator of the BARB project. “If we ‘aim-and-shoot’ using a radioactive beam and PET imaging, we can see where the beam is and can then correct it. By doing this, we can reduce the margins around the target that spoil the precision of particle therapy.”
In vivo experiments
To test this premise, Durante and colleagues performed in vivo experiments at the GSI/FAIR accelerator facility in Darmstadt. For online range verification, they used a portable small-animal in-beam PET scanner built by Katia Parodi and her team at LMU Munich. The scanner, initially designed for the ERC project SIRMIO (Small-animal proton irradiator for research in molecular image-guided radiation-oncology), contains 56 depth-of-interaction detectors – based on scintillator blocks of pixelated LYSO crystals – arranged spherically with an inner diameter of 72 mm.

“Not only does our spherical in-beam PET scanner offer unprecedented sensitivity and spatial resolution, but it also enables on-the-fly monitoring of the activity implantation for direct feedback during irradiation,” says Parodi, co-principal investigator of the BARB project.
The researchers used a radioactive 11C-ion beam – produced at the GSI fragment separator – to treat 32 mice with an osteosarcoma tumour implanted in the neck near the spinal cord. To encompass the full target volume, they employed a range modulator to produce a spread-out Bragg peak (SOBP) and a plastic compensator collar, which also served to position and immobilize the mice. The anaesthetized animals were placed vertically inside the PET scanner and treated with either 20 or 5 Gy at a dose rate of around 1 Gy/min.
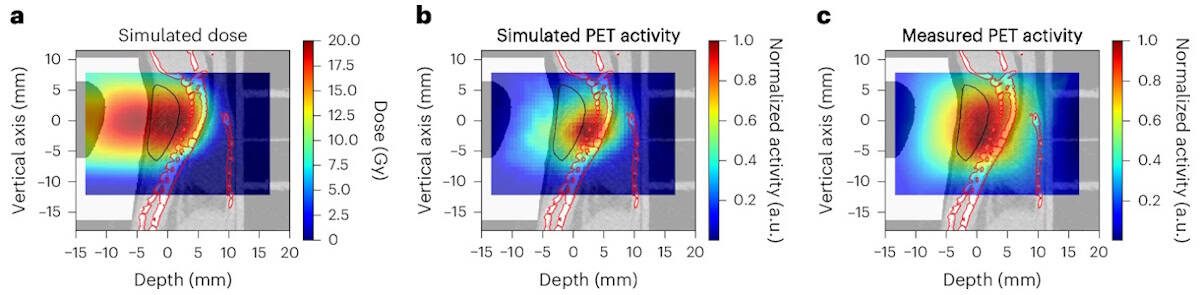
For each irradiation, the team compared the measured activity with Monte Carlo-simulated activity based on pre-treatment microCT scans. The activity distributions were shifted by about 1 mm, attributed to anatomical changes between the scans (with mice positioned horizontally) and irradiation (vertical positioning). After accounting for this anatomical shift, the simulation accurately matched the measured activity. “Our findings reinforce the necessity of vertical CT planning and highlight the potential of online PET as a valuable tool for upright particle therapy,” the researchers write.
With the tumour so close to the spine, even small range uncertainties risk damage to the spinal cord, so the team used the online PET images generated during the irradiation to check that the SOPB did not cover the spine. While this was not seen in any of the animals, Durante notes that if it had, the beam could be moved to enable “truly adaptive” particle therapy. Assessing the mice for signs of radiation-induced myelopathy (which can lead to motor deficits and paralysis) revealed that no mice exhibited severe toxicity, further demonstrating that the spine was not exposed to high doses.
Following treatment, tumour measurements revealed complete tumour control after 20 Gy irradiation and prolonged tumour growth delay after 5 Gy, suggesting complete target coverage in all animals.
The researchers also assessed the washout of the signal from the tumour, which includes a slow activity decrease due to the decay of 11C (which has a half-life of 20.34 min), plus a faster decrease as blood flow removes the radioactive isotopes from the tumour. The results showed that the biological washout was dose-dependent, with the fast component visible at 5 Gy but disappearing at 20 Gy.
“We propose that this finding is due to damage to the blood vessel feeding the tumour,” says Durante. “If this is true, high-dose radiotherapy may work in a completely different way from conventional radiotherapy: rather than killing all the cancer stem cells, we just starve the tumour by damaging the blood vessels.”
Future plans
Next, the team intends to investigate the use of 10C or 15O treatment beams, which should provide stronger signals and increased temporal resolution. A new Super-FRS fragment separator at the FAIR accelerator facility will provide the high-intensity beams required for studies with 10C.
Looking further ahead, clinical translation will require a realistic and relatively cheap design, says Durante. “CERN has proposed a design [the MEDICIS-Promed project] based on ISOL [isotope separation online] that can be used as a source of radioactive beams in current accelerators,” he tells Physics World. “At GSI we are also working on a possible in-flight device for medical accelerators.”
The findings are reported in Nature Physics.
The post Radioactive ion beams enable simultaneous treatment and imaging in particle therapy appeared first on Physics World.

